Q.
This question has been submitted in the following subject: science A body of water already at equilbrium contains 10^-5 mol/litter of carbonic acid, H2CO3. a)What is the concentration of bicarbonate, HCO3^-, at pH values of 4, 7, and 10. b)Use the same pH conditions in part a) but in seawater having an ionic strength of 0.5M. (Because pH is properly defined on the basis of hydrogen activity, ionic strength corrections need only be made for HCO3^-.) Not sure what to do. There should be an answer for each pH value.
ANSWER:
(a)
Ki = [H+][HCO3-] / [H2CO3] = 4.3x10^-7 (from a table of Ka values)
For a pH of 4, [H+] = 1x10^-4
[1x10^-4][HCO3-] / [(1x10^-5)] = 4.3x10^-7
[HCO3-] = [H2CO3](Ka) / [H+]
[HCO3-] = (1x10^-5)(4.3x10^-7) / (1x10^-4) = 4.3x10^-8
You find [HCO3-] for other pH’s in a similar way. As the pH rises, the concentration of HCO3- should also rise.
(b)
Reference:
http://www.chembuddy.com/?left=pH-calculation&right=ionic-strength-activity-coefficients
The hydrogen activity coefficient can be calculated by:
Log(f) = [(-.51z^2)*sqrt(I)] / [1 + sqrt(I)] z = ionic charge, I = ionic strength
Log(f) = -0.21125 and f = 0.615
The hydrogen activity is
a = (f)[H+]
For pH = 4,
a = {H+} = (0.615)(1x10^-4) = 6.15x10^-5 <--- H+ activity replacing [H+]
[HCO3-] = [H2CO3](Ka) / [H+]
[HCO3-] = (1x10^-5)(4.3x10^-7) / (6.15x10^-5) = 7.0x10^-8
For other pH’s, do similar calculations with the same activity coefficient.
=======================
11/30/07
Photosynthesis Calculations
Q.
In photosynthesis, CO2 (g) combines with H2O (g) to form glucose and oxygen. How many moles of glucose would be produced if a) 5 mol of CO2 (g) were used? b) 3 mol of H2O (g) were used? c) 18 mol of O2 (g) were released. My teacher told me to set this up as a math ratio to figure it out: 6 mol CO2 (g) = 5 mol CO2 (g) 1 mol C6H12O6 (s) ? C6H12O6 (s)
A.
The chemical equation is:
6CO2 + 6H2O --–> C6H12O6 + 6O2
(a) (5 mol CO2)( 1 mol C6H12O6 / 6 mol CO2) = 5/6 moles C6H12O6 or 0.833 mol CO2
(Moles CO2 canceling out, mole C6H12O6 remains)
(b) (3 mol. H2O)(1 mol.C6H12O6 / 6 mol.H2O) = 0.5moles C6H12O6
(c) (18 mol O2)(1 mol C6H12O6 / 6 mol O2) = 3 moles C6H12O6
==============
In photosynthesis, CO2 (g) combines with H2O (g) to form glucose and oxygen. How many moles of glucose would be produced if a) 5 mol of CO2 (g) were used? b) 3 mol of H2O (g) were used? c) 18 mol of O2 (g) were released. My teacher told me to set this up as a math ratio to figure it out: 6 mol CO2 (g) = 5 mol CO2 (g) 1 mol C6H12O6 (s) ? C6H12O6 (s)
A.
The chemical equation is:
6CO2 + 6H2O --–> C6H12O6 + 6O2
(a) (5 mol CO2)( 1 mol C6H12O6 / 6 mol CO2) = 5/6 moles C6H12O6 or 0.833 mol CO2
(Moles CO2 canceling out, mole C6H12O6 remains)
(b) (3 mol. H2O)(1 mol.C6H12O6 / 6 mol.H2O) = 0.5moles C6H12O6
(c) (18 mol O2)(1 mol C6H12O6 / 6 mol O2) = 3 moles C6H12O6
==============
Salt Formation from Oxides
Q.
What salts are produced when you mix oxides with hydrochloric acid, sulphuric acid and nitric acid?
A.
The salts produced are chlorides from HCl, sulfates from H2SO4, and nitrates from HNO3 when the acid reacts with a metal oxide. Examples:
MgO + 2HCl ---> CaCl2 + H2O (magnesium chloride)
MgO + H2SO4 ---> CaSO4 + H2O (magnesium sulfate)
MgO + 2HNO3 ---> Mg(NO3)2 + H2O (magnesium nitrate)
==========
What salts are produced when you mix oxides with hydrochloric acid, sulphuric acid and nitric acid?
A.
The salts produced are chlorides from HCl, sulfates from H2SO4, and nitrates from HNO3 when the acid reacts with a metal oxide. Examples:
MgO + 2HCl ---> CaCl2 + H2O (magnesium chloride)
MgO + H2SO4 ---> CaSO4 + H2O (magnesium sulfate)
MgO + 2HNO3 ---> Mg(NO3)2 + H2O (magnesium nitrate)
==========
Molarity from Grams and Volume of Solution
Q.
You have 10.0 g of NaCl (58.43g/mol) and dissolve it in 0.5L of water. What is the molarity of the resulting solution.
A.
(a) Convert the 10.0g NaCl to moles: (10.0g) / 58.43g/mol = 0.171 moles NaCl
(b) 0.171 moles NaCl / (0.5L) = 0.342 mol/L
============
You have 10.0 g of NaCl (58.43g/mol) and dissolve it in 0.5L of water. What is the molarity of the resulting solution.
A.
(a) Convert the 10.0g NaCl to moles: (10.0g) / 58.43g/mol = 0.171 moles NaCl
(b) 0.171 moles NaCl / (0.5L) = 0.342 mol/L
============
Carbon Footprint Calculations
Q.
How can I find out about carbon footprints as the sites I have been on to don't give me the infomation that i need. the question is how does a carbon footprint work and what are the effects of globel warming (green house affect)
A.
“A carbon footprint is a measure of the impact human activities have on the environment in terms of the amount of green house gases produced, measured in units of carbon dioxide.” That is the definition given by the online encyclopedia Wikipedia.
Your carbon footprint depends on your location and your lifestyle. Every appliance and product you use adds carbon dioxide and other greenhouse gases to the air. There are several online carbon footprint calculators. Here are some:
http://www.safeclimate.net/calculator/
http://www.climatecrisis.net/takeaction/carboncalculator/
http://www.bp.com/extendedsectiongenericarticle.do?categoryId=9015627&contentId=7029058
The higher your carbon footprint, the more your contribute toward global warming. The link below explains how the carbon footprint calculations are done:
http://www.carboncalculator.co.uk/explanation.php
How can I find out about carbon footprints as the sites I have been on to don't give me the infomation that i need. the question is how does a carbon footprint work and what are the effects of globel warming (green house affect)
A.
“A carbon footprint is a measure of the impact human activities have on the environment in terms of the amount of green house gases produced, measured in units of carbon dioxide.” That is the definition given by the online encyclopedia Wikipedia.
Your carbon footprint depends on your location and your lifestyle. Every appliance and product you use adds carbon dioxide and other greenhouse gases to the air. There are several online carbon footprint calculators. Here are some:
http://www.safeclimate.net/calculator/
http://www.climatecrisis.net/takeaction/carboncalculator/
http://www.bp.com/extendedsectiongenericarticle.do?categoryId=9015627&contentId=7029058
The higher your carbon footprint, the more your contribute toward global warming. The link below explains how the carbon footprint calculations are done:
http://www.carboncalculator.co.uk/explanation.php
Molarity and Moles
Q.
How many moles are there in 500cm3 of 5.0M lithium bromide, NaBr, solution?
A.
Moles = Molarity x Liters
or
n = VM
1 cm^3 = 1 mL, 1 L = 1000 mL, and M = mol/L
500 cm^3 = 500 mL = 0.500 L
moles = (5.0 mol/L)(0.500 L) = 2.5 moles NaBr
===========
How many moles are there in 500cm3 of 5.0M lithium bromide, NaBr, solution?
A.
Moles = Molarity x Liters
or
n = VM
1 cm^3 = 1 mL, 1 L = 1000 mL, and M = mol/L
500 cm^3 = 500 mL = 0.500 L
moles = (5.0 mol/L)(0.500 L) = 2.5 moles NaBr
===========
Molarity Calculation
Q.
What is the molarity of 250cm3 sodium chloride solution containing 2 moles NaCl?
A.
Molarity = moles / liters
(1) Convert 250 cm^3 to liters: (250 mL)(1 L / 1000 mL) = 0.250 L (NOTE: 1 mL = 1 cm^3)
(2) Divide 2.0 moles by the number of liters: 2 mol. / 0.250 L = 8.0 mol/L
=======
What is the molarity of 250cm3 sodium chloride solution containing 2 moles NaCl?
A.
Molarity = moles / liters
(1) Convert 250 cm^3 to liters: (250 mL)(1 L / 1000 mL) = 0.250 L (NOTE: 1 mL = 1 cm^3)
(2) Divide 2.0 moles by the number of liters: 2 mol. / 0.250 L = 8.0 mol/L
=======
Q.
Right now i am in the Waves section of our textbook. Which contains the Electromagnetic Spectrum and so on. I don't get any of it so i don't have a specific question.
A.
How you study electromagnetic radiation depends on the grade level and class you are taking. It makes a big difference whether you are in a General Science class or a Physics class. Either way, you must spend some time studying the topic and understand it enough on your own before you can ask the right questions. Here are a few links that give you a general idea about your topic. I hope one of them will give you enough understanding to get started:
http://praxis.pha.jhu.edu/science/emspec.html
http://hyperphysics.phy-astr.gsu.edu/hbase/ems3.htm
http://amazing-space.stsci.edu/resources/explorations/light/ems-frames.html
http://www.yorku.ca/eye/spectru.htm
http://www.electro-optical.com/bb_rad/emspect.htm
============
Right now i am in the Waves section of our textbook. Which contains the Electromagnetic Spectrum and so on. I don't get any of it so i don't have a specific question.
A.
How you study electromagnetic radiation depends on the grade level and class you are taking. It makes a big difference whether you are in a General Science class or a Physics class. Either way, you must spend some time studying the topic and understand it enough on your own before you can ask the right questions. Here are a few links that give you a general idea about your topic. I hope one of them will give you enough understanding to get started:
http://praxis.pha.jhu.edu/science/emspec.html
http://hyperphysics.phy-astr.gsu.edu/hbase/ems3.htm
http://amazing-space.stsci.edu/resources/explorations/light/ems-frames.html
http://www.yorku.ca/eye/spectru.htm
http://www.electro-optical.com/bb_rad/emspect.htm
============
Sound Reproduction
Q.
I need to write a report on the elements of recording and processing sound. I have looked on many websites and i cannot find the technical process of recording and playing back sound. (from microphone to speaker) If you have any idea or know of a website that can tell me i would be very grateful
A.
The Howstuffworks web site explains how sound waves are converted to electrical current fluctuations through a microphone and back to sound through a speaker. Go to:
http://electronics.howstuffworks.com/question309.htm
http://electronics.howstuffworks.com/speaker11.htm
=========
I need to write a report on the elements of recording and processing sound. I have looked on many websites and i cannot find the technical process of recording and playing back sound. (from microphone to speaker) If you have any idea or know of a website that can tell me i would be very grateful
A.
The Howstuffworks web site explains how sound waves are converted to electrical current fluctuations through a microphone and back to sound through a speaker. Go to:
http://electronics.howstuffworks.com/question309.htm
http://electronics.howstuffworks.com/speaker11.htm
=========
Gas Variables
Q.
As the pressure of a fixed mass of a gas is increased at constant temperature, the density of the gas (increases, decreases, remains the same).
A.
The Ideal Gas Law, PV = nRT can be changed to PV = (mass/formula mass)RT. That can be rearranged to:
(P)(formula mass) = )(mass/V)RT or:
P*(formula mass) = DRT. This shows that pressure is proportional to density (they both increase at the same ratio).
Q.
As the absolute temperature of a fixed mass of an ideal gas is increased at constant pressure, the volume occupied by the gas (increases, decreases, remains the same).
A.
Again, PV = nRT. Volume is proportional to absolute temperature.
Q.
The absolute temperature of a fixed mass of ideal gas is tripled while its volume remains constant. The ratio of the final pressure of the gas to its initial pressure is (3 to 1, 1 to 1, 1.5 to 1, or 9 to 1) I think it is 3 to 1.
A.
P1V1/T1 = P2V2/T2
If V1 = V2, the above formula becomes: P1/T1 = P2/T2 or by rearranging, P2/P1 = T2/T1
That last relationship shows that at constant volume, the pressure ratio equals the temperature ratio
As the pressure of a fixed mass of a gas is increased at constant temperature, the density of the gas (increases, decreases, remains the same).
A.
The Ideal Gas Law, PV = nRT can be changed to PV = (mass/formula mass)RT. That can be rearranged to:
(P)(formula mass) = )(mass/V)RT or:
P*(formula mass) = DRT. This shows that pressure is proportional to density (they both increase at the same ratio).
Q.
As the absolute temperature of a fixed mass of an ideal gas is increased at constant pressure, the volume occupied by the gas (increases, decreases, remains the same).
A.
Again, PV = nRT. Volume is proportional to absolute temperature.
Q.
The absolute temperature of a fixed mass of ideal gas is tripled while its volume remains constant. The ratio of the final pressure of the gas to its initial pressure is (3 to 1, 1 to 1, 1.5 to 1, or 9 to 1) I think it is 3 to 1.
A.
P1V1/T1 = P2V2/T2
If V1 = V2, the above formula becomes: P1/T1 = P2/T2 or by rearranging, P2/P1 = T2/T1
That last relationship shows that at constant volume, the pressure ratio equals the temperature ratio
Mole Ratio Stoichiometry
Q.
How many moles of hydrogen gas are produced when 0.1 moles of sodium reacts with 0.1 moles of water?
[2Na(s) + 2H2O (l)>>>> H2(g) + 2NaOH(aq)]?
A.
2 moles of Na react with 2 moles of H2O, producing 1 mole of H2 as the chemical equation shows.
Based on the coefficients of the chemical equation, the ratio of (2 : 2 : 1) is the same as
(1 : 1 : 0.5) or (0.1 : 0.1 : 05)
2Na + 2H2O ---> H2 + 2NaOH
1Na + 1H2O ---> 0.5H2 + 1NaOH
0.1Na + 0.1H2O --->0.05H2 + 0.1NaOH
All three chemical equations show the same ratio with different coefficients. The last one has the answer to your question.
A more formal solution using “conversion factors” is:
(0.1 mole Na)(1 mol H2 / 2 mol Na) = 0.05 mol H2
or
(0.1 mole H2O)(1 mol H2 / 2 mol H2O) = 0.05 mol H2
If the last two solutions were not the same, you should have taken the smaller one since that one is automatically based on the “limiting reagent”. In this case both calculation both solutions give the same answer since Na and H2O are used at the same ratio as the one shown in the chemical equation.
=========
How many moles of hydrogen gas are produced when 0.1 moles of sodium reacts with 0.1 moles of water?
[2Na(s) + 2H2O (l)>>>> H2(g) + 2NaOH(aq)]?
A.
2 moles of Na react with 2 moles of H2O, producing 1 mole of H2 as the chemical equation shows.
Based on the coefficients of the chemical equation, the ratio of (2 : 2 : 1) is the same as
(1 : 1 : 0.5) or (0.1 : 0.1 : 05)
2Na + 2H2O ---> H2 + 2NaOH
1Na + 1H2O ---> 0.5H2 + 1NaOH
0.1Na + 0.1H2O --->0.05H2 + 0.1NaOH
All three chemical equations show the same ratio with different coefficients. The last one has the answer to your question.
A more formal solution using “conversion factors” is:
(0.1 mole Na)(1 mol H2 / 2 mol Na) = 0.05 mol H2
or
(0.1 mole H2O)(1 mol H2 / 2 mol H2O) = 0.05 mol H2
If the last two solutions were not the same, you should have taken the smaller one since that one is automatically based on the “limiting reagent”. In this case both calculation both solutions give the same answer since Na and H2O are used at the same ratio as the one shown in the chemical equation.
=========
11/16/07
Radioactive Decay - Mass remaining
Q.
Polonium-210 is a radioactive element with a half-life of 20 weeks. From a sample of 25 g, how much would remain after 30 weeks?
A.
ln(M/Mo) = -kt (M = mass left, Mo = original mass)
k = 0.693/T(half) = 0.693/20 = 0.03465
ln(M/25) = - (0.03465)(30wks) = -1.0395
lnM - ln25 = -1.0395
lnM = ln25 -1.0395 = 2.1794
M = 8.84 g radioactive material remaining
==============
Polonium-210 is a radioactive element with a half-life of 20 weeks. From a sample of 25 g, how much would remain after 30 weeks?
A.
ln(M/Mo) = -kt (M = mass left, Mo = original mass)
k = 0.693/T(half) = 0.693/20 = 0.03465
ln(M/25) = - (0.03465)(30wks) = -1.0395
lnM - ln25 = -1.0395
lnM = ln25 -1.0395 = 2.1794
M = 8.84 g radioactive material remaining
==============
11/12/07
Angular and Linear Velocity
Q.
A bicycle wheel rotates 200 revolutions per minute. The tire tread is 21 in. from the center of the tire. find to the nearest hundreth for a point on the tire its: (a)angular velocity (b) linear velocity.
A.
1 revolution = 2π radians
The circumference = (2π rad.)(21 in./rad) = 131.95 in.
The angular velocity is:
(200 rev / min)(2π rad/rev) = 400π rad/min ---> 6.6667π rad/s ---> 20.944 rad/s
The linear velocity is:
(400π rad/min)(21 in/rad) = 26389.4 in/min ---> 2199.1 ft/min ---> 36.652 ft/min
================
A bicycle wheel rotates 200 revolutions per minute. The tire tread is 21 in. from the center of the tire. find to the nearest hundreth for a point on the tire its: (a)angular velocity (b) linear velocity.
A.
1 revolution = 2π radians
The circumference = (2π rad.)(21 in./rad) = 131.95 in.
The angular velocity is:
(200 rev / min)(2π rad/rev) = 400π rad/min ---> 6.6667π rad/s ---> 20.944 rad/s
The linear velocity is:
(400π rad/min)(21 in/rad) = 26389.4 in/min ---> 2199.1 ft/min ---> 36.652 ft/min
================
11/11/07
Reaction Stoichiometry with Gases
Q.
(a) The electroylsis of water produces hydrogen and oxygen gases as shown in the following equation 2H2O to give 2H2+ O2 How many moles of water are reacted if 5.00ml of water is electrolyzed.
(b)If the gases produced from the 5.00ml of water are collected in a 5.00L flask at 22 degrees what will be the total pressure in the flask?
(c)What is the partial pressure of each gas?
A.
(a)
(5.00mL)(1.00 g/mL) = 5.00 g 2H2O
(5.00g)(1 mol / 18.0g) = 0.28 moles 2H2O
(b)
(0.28 mol 2H2O)(3 mol gas / 2 mol H2) = 0.417 moles of gas
PV=nRT
P = nRT/V
P = (0.417 mol)(0.0821L.atm./K.mol)(295K) / (5.0L) = 2.02 atm
(c)
P(O2) = (1 mol O2)/(3 mol gas)(2.02 atm) = 0.673 atm.
P(H2) = (2 mol H2)/(3 mol gas)2.02 atm) = 1.347 atm.
=============
(a) The electroylsis of water produces hydrogen and oxygen gases as shown in the following equation 2H2O to give 2H2+ O2 How many moles of water are reacted if 5.00ml of water is electrolyzed.
(b)If the gases produced from the 5.00ml of water are collected in a 5.00L flask at 22 degrees what will be the total pressure in the flask?
(c)What is the partial pressure of each gas?
A.
(a)
(5.00mL)(1.00 g/mL) = 5.00 g 2H2O
(5.00g)(1 mol / 18.0g) = 0.28 moles 2H2O
(b)
(0.28 mol 2H2O)(3 mol gas / 2 mol H2) = 0.417 moles of gas
PV=nRT
P = nRT/V
P = (0.417 mol)(0.0821L.atm./K.mol)(295K) / (5.0L) = 2.02 atm
(c)
P(O2) = (1 mol O2)/(3 mol gas)(2.02 atm) = 0.673 atm.
P(H2) = (2 mol H2)/(3 mol gas)2.02 atm) = 1.347 atm.
=============
Chemistry Dictionary
Where can I find a good chemistry dictionary thats fast and would have easy things like molecule to hard things like Aufbau Principle and Hund's Rule?
A.
None of the online chemistry dictionaries I found are comprehensive enough to include those terms you mentioned. For the easier terms (such as your first two) I found very good definitions on Encyclopedia Britannica Online at:
http://www.britannica.com/
For Hund's Rule, I had to use the Google search engine:
http://www.google.com
The best place to learn chemistry terminology is from a good General Chemistry textbook. There you will find those terms used in the proper context. For example, the chapter (or unit) on atomic structure will tell you all about the Aufbau Principle and Hund's Rule in the section about Electron Configurations. Also you will be given exercises and problems that use those terms. That will help you understand them. A dictionary is of very limited value in studying chemistry.
===============
A.
None of the online chemistry dictionaries I found are comprehensive enough to include those terms you mentioned. For the easier terms (such as your first two) I found very good definitions on Encyclopedia Britannica Online at:
http://www.britannica.com/
For Hund's Rule, I had to use the Google search engine:
http://www.google.com
The best place to learn chemistry terminology is from a good General Chemistry textbook. There you will find those terms used in the proper context. For example, the chapter (or unit) on atomic structure will tell you all about the Aufbau Principle and Hund's Rule in the section about Electron Configurations. Also you will be given exercises and problems that use those terms. That will help you understand them. A dictionary is of very limited value in studying chemistry.
===============
11/10/07
Heat of Combustion Calculation
Q.
A chemical engineer studying the properties of fuel placed 1.988 g of hydrocarbon in the bomb of a calorimeter and filled it with O2 gas. The bomb was immersed in 2.321 L water and the reaction initiated. The water temp rose from 21.06 to 27.80 degrees Celsius. If the calorimete (xcluding the H2O) had a heat capacity of 480 J/K, what was the heat of combustion of the fuel?
A
Total Heat = (4.184 J/g•C)(2321 g)(27.80 C - 21.06 C) + (480 J/C)(27.80 C - 21.06 C) = ______ J
==============
A chemical engineer studying the properties of fuel placed 1.988 g of hydrocarbon in the bomb of a calorimeter and filled it with O2 gas. The bomb was immersed in 2.321 L water and the reaction initiated. The water temp rose from 21.06 to 27.80 degrees Celsius. If the calorimete (xcluding the H2O) had a heat capacity of 480 J/K, what was the heat of combustion of the fuel?
A
Total Heat = (4.184 J/g•C)(2321 g)(27.80 C - 21.06 C) + (480 J/C)(27.80 C - 21.06 C) = ______ J
==============
Energy in a Gallon of Octane
Q.
Pure liquid octane (C8H18) (d= 0.702 g/ml) is used as the fuel in a test of a new automobile drive train. How much energy is released by the complete combustion of all the octane in a 21.) gal fuel tank (Hcomb= -5.45 E 3 kJ/mol)?
A.
You must convert the gallons of octane to grams:
(21.0 gal)(3.785 L/1 gal )(1000 mL/1 L )(0.702 g/ 1 mL) = 55800 grams
1 mole of C8H18 = 114.23 g
Convert grams to moles:
(55800 g)(1 mol / 114.23 g) = 488.5 moles
Heat = ( 488.5 mol)(5450 kJ/mol) = 2.66x10^6 kJ
======================
Pure liquid octane (C8H18) (d= 0.702 g/ml) is used as the fuel in a test of a new automobile drive train. How much energy is released by the complete combustion of all the octane in a 21.) gal fuel tank (Hcomb= -5.45 E 3 kJ/mol)?
A.
You must convert the gallons of octane to grams:
(21.0 gal)(3.785 L/1 gal )(1000 mL/1 L )(0.702 g/ 1 mL) = 55800 grams
1 mole of C8H18 = 114.23 g
Convert grams to moles:
(55800 g)(1 mol / 114.23 g) = 488.5 moles
Heat = ( 488.5 mol)(5450 kJ/mol) = 2.66x10^6 kJ
======================
Why Study Chemistry?
Q.
Y is chemistry so important? not all of us are gonna be scientist email me ur answers.
ps. u probbly thinkin i must b the one gettin bad grades, no im not im jus wondering.
A.
You may not be planning to make your living as a scientist but you will be dealing with science and technical issues all your life: The food you eat, the medicines you take, the sources of energy you use (gasoline, natural gas, electricity, etc.); how you use, and how much you pay for those things. If you are running or using a backyard swimming pool you have to know how to test for chlorine and for acidity otherwise you will have too much or too little chlorine in the water. Could come up with many more illustrations like that just for around the house.
If you are a trial lawyer you will be dealing with physical and chemical evidence. If you are a political leader you will be dealing with many industrial and environmental issues. "Experts" don't always agree on a technical issue. So you must know enough science to decide which scientist is best qualified and most objective on a particular question.
The facts you learn in a science class are not as important as learning to use them in solving problems. If you learn nothing else in your chemistry class, you will learn how to study, how to think, and how to stick to a task until it is finished. I suspect that chemistry is made a prerequisite in many non-science areas just to select those who have the study skills, thinking skills, and perseverance to be successful.
None of the practical reasons I gave you for studying chemistry are really enough. Curiosity about the world around you is the best reason and the best motivator. No subject is really all that interesting while you build those basic skills. But if you hang in there until you have the basic skills, then you are free to pursue something that is really original and interesting. Develop your curiosity and the rest will work itself out.
===================
Y is chemistry so important? not all of us are gonna be scientist email me ur answers.
ps. u probbly thinkin i must b the one gettin bad grades, no im not im jus wondering.
A.
You may not be planning to make your living as a scientist but you will be dealing with science and technical issues all your life: The food you eat, the medicines you take, the sources of energy you use (gasoline, natural gas, electricity, etc.); how you use, and how much you pay for those things. If you are running or using a backyard swimming pool you have to know how to test for chlorine and for acidity otherwise you will have too much or too little chlorine in the water. Could come up with many more illustrations like that just for around the house.
If you are a trial lawyer you will be dealing with physical and chemical evidence. If you are a political leader you will be dealing with many industrial and environmental issues. "Experts" don't always agree on a technical issue. So you must know enough science to decide which scientist is best qualified and most objective on a particular question.
The facts you learn in a science class are not as important as learning to use them in solving problems. If you learn nothing else in your chemistry class, you will learn how to study, how to think, and how to stick to a task until it is finished. I suspect that chemistry is made a prerequisite in many non-science areas just to select those who have the study skills, thinking skills, and perseverance to be successful.
None of the practical reasons I gave you for studying chemistry are really enough. Curiosity about the world around you is the best reason and the best motivator. No subject is really all that interesting while you build those basic skills. But if you hang in there until you have the basic skills, then you are free to pursue something that is really original and interesting. Develop your curiosity and the rest will work itself out.
===================
Molecular Orbital Energy Levels
Q.
What is the sequence of molecular orbitals from lower to higher energies? How do you find the bonding order in a small diatomic molecule?
A.
The energy sequence is:
σ1s2, σ1s*2, σ2s2, σ2s*2, π2py2, π2pz2, σ2p2, π2py*2, π2pz*2, σ2p*2
See diagram in
Molecular Orbital Theory
To find the bonding order:
1. Place all the electrons in the diatomic molecule in orbitals following the above sequence (maximum of 2 per orbital)
2. Count the total number of electrons in NONstar molecular orbitals.
3. Count the total number of electrons in star orbitals.
Bonding order = (nonstar total - star total) / 2
What is the sequence of molecular orbitals from lower to higher energies? How do you find the bonding order in a small diatomic molecule?
A.
The energy sequence is:
σ1s2, σ1s*2, σ2s2, σ2s*2, π2py2, π2pz2, σ2p2, π2py*2, π2pz*2, σ2p*2
See diagram in
Molecular Orbital Theory
To find the bonding order:
1. Place all the electrons in the diatomic molecule in orbitals following the above sequence (maximum of 2 per orbital)
2. Count the total number of electrons in NONstar molecular orbitals.
3. Count the total number of electrons in star orbitals.
Bonding order = (nonstar total - star total) / 2
Molecular orbitals of H2 and H2+
Q.
Use a molecular-orbital analysis to predict which species in each of the following pairs has the stronger bonding between atoms.
A.
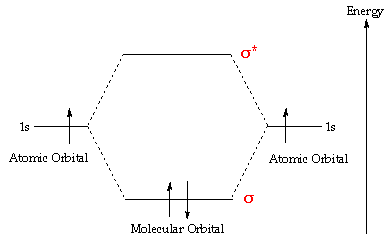
The s-orbitals of the two H atoms merge to form one bonding orbital, sigma-1 and one antibonding orbital, sigma-1*. The strongest bonding between the two hydrogen atoms exists when we have 2 electrons in the sigma-1 bonding orbital and no electrons in the sigma-1* orbital.
The H 2 molecule has 2 electrons in the sigma-1s bonding orbital which fills before the sigma-1s* orbital which has higher energy. That is equivalent ot 1 whole covalent bond.
The H 2+ molecule has only 1 electron which goes into the sigma-1s bonding orbital and no electrons in sigma-1s*. That is equivalent to half a covalent bond. That should explain why the H 2 molecule is more stable than the H 2+ ion.
For more on molecular orbitals of diatomic molecules and ions, go to:
http://www.sparknotes.com/chemistry/bonding/molecularorbital/section1.html
http://www.ch.ic.ac.uk/vchemlib/course/mo_theory/main.html
==============
Use a molecular-orbital analysis to predict which species in each of the following pairs has the stronger bonding between atoms.
A.

The s-orbitals of the two H atoms merge to form one bonding orbital, sigma-1 and one antibonding orbital, sigma-1*. The strongest bonding between the two hydrogen atoms exists when we have 2 electrons in the sigma-1 bonding orbital and no electrons in the sigma-1* orbital.
The H 2 molecule has 2 electrons in the sigma-1s bonding orbital which fills before the sigma-1s* orbital which has higher energy. That is equivalent ot 1 whole covalent bond.
The H 2+ molecule has only 1 electron which goes into the sigma-1s bonding orbital and no electrons in sigma-1s*. That is equivalent to half a covalent bond. That should explain why the H 2 molecule is more stable than the H 2+ ion.
For more on molecular orbitals of diatomic molecules and ions, go to:
http://www.sparknotes.com/chemistry/bonding/molecularorbital/section1.html
http://www.ch.ic.ac.uk/vchemlib/course/mo_theory/main.html
==============
Bond Polarity
Q.
which of these bonds is most polar and which is least: S-O, Cl-Cl or Cl-O?Justify your answer.
A.
Look up the electronegativity values for sulfur, chlorine, and oxygen. The bond with the biggest *difference* in electronegativity values is the most polar.
Electronegativity Table
==================
which of these bonds is most polar and which is least: S-O, Cl-Cl or Cl-O?Justify your answer.
A.
Look up the electronegativity values for sulfur, chlorine, and oxygen. The bond with the biggest *difference* in electronegativity values is the most polar.
==================
Limiting Reagent - Solution Outline
Q.
For the balanced equation shown below, if 95.3 grams of C4H9N were reacted with 220 grams of O2, how many grams of H2O would be produced?
A.
4C4H9N + 29O2 => 16CO2 + 18H2O + 4NO2
1. Calculate the molar masses of the three compounds:
*The molar mass of C4H9N is: (12.011)(4) + (1.00793)(9) + (14.007) = 71.183 g/mol
*The molar mass of O2 = 32.000 g/mol
*The molar mass of H2O = (1.00793)(2) + 16.000 = 18.0153 g/mol
2. Calculate the number of moles of the two reactants:
*(95.3g C4H9N)(1 mol C4H9N / 71.183 g/mol.C4H9N) = 1.339 mol C4H9N
*(220g O2)(1 mol O2 / 32.000 g O2) = 6.875 mol O2
3. Calculate the moles of C4H9N *needed* to react with 6.875 mol O2:
*(6.875 mol O2)(4 mol C4H9N / 29mol O2) = 0.9483 C4H9N [NOTE: The 4 and the 29 are coefficients from the BALANCED chemical equation]
4. *Compare* the moles of C4H9N present (1.339) to moles needed (0.9483). As you can see, we have more than the number of moles needed. That means the C4H9N is the *excess reagent* and O2 is the *limiting reagent*.
5. All the above procedure does is give us the LIMITING reagent, O2. We use the moles of the limiting reagent to get the amount of product:
*(6.875 mol O2)(18 mol H2O / 29 mol O2) = 4.27 moles H2O
6. Multiply the number of moles of H2O by the molar mass of H2O to get the grams of H2O produced.
==================
For the balanced equation shown below, if 95.3 grams of C4H9N were reacted with 220 grams of O2, how many grams of H2O would be produced?
A.
4C4H9N + 29O2 => 16CO2 + 18H2O + 4NO2
1. Calculate the molar masses of the three compounds:
*The molar mass of C4H9N is: (12.011)(4) + (1.00793)(9) + (14.007) = 71.183 g/mol
*The molar mass of O2 = 32.000 g/mol
*The molar mass of H2O = (1.00793)(2) + 16.000 = 18.0153 g/mol
2. Calculate the number of moles of the two reactants:
*(95.3g C4H9N)(1 mol C4H9N / 71.183 g/mol.C4H9N) = 1.339 mol C4H9N
*(220g O2)(1 mol O2 / 32.000 g O2) = 6.875 mol O2
3. Calculate the moles of C4H9N *needed* to react with 6.875 mol O2:
*(6.875 mol O2)(4 mol C4H9N / 29mol O2) = 0.9483 C4H9N [NOTE: The 4 and the 29 are coefficients from the BALANCED chemical equation]
4. *Compare* the moles of C4H9N present (1.339) to moles needed (0.9483). As you can see, we have more than the number of moles needed. That means the C4H9N is the *excess reagent* and O2 is the *limiting reagent*.
5. All the above procedure does is give us the LIMITING reagent, O2. We use the moles of the limiting reagent to get the amount of product:
*(6.875 mol O2)(18 mol H2O / 29 mol O2) = 4.27 moles H2O
6. Multiply the number of moles of H2O by the molar mass of H2O to get the grams of H2O produced.
==================
Hydrogen Atom - Electron Transitions
Q.
calculate the wavelength of light emitted when each of the following transitions occur in the hydrogen atom. n=5---n=3
A.
The energy of a hydrogen electron at the nth energy level is:
En = -B/n^2
where B = 2.179x10^-18 J, a constant
The energy of the transition is:
E = E5 - E3
or
E = [(-2.179x10^-18 J) / 5^2] - [(-2.179x10^-18 J) / 3^2] = ______ ?
After you find the transition energy, E, you calculate the frequency, f, using, E = hf, or
E = (6.626x10^-34 Js)(f)
or
f = E / (6.626x10^-34 Js)
Finally,
Use
(wavelength)(frequency) = (3.00x10^8m/s)
or
wavelength = (3.00x10^8m/s) / freq. = ______?
================
calculate the wavelength of light emitted when each of the following transitions occur in the hydrogen atom. n=5---n=3
A.
The energy of a hydrogen electron at the nth energy level is:
En = -B/n^2
where B = 2.179x10^-18 J, a constant
The energy of the transition is:
E = E5 - E3
or
E = [(-2.179x10^-18 J) / 5^2] - [(-2.179x10^-18 J) / 3^2] = ______ ?
After you find the transition energy, E, you calculate the frequency, f, using, E = hf, or
E = (6.626x10^-34 Js)(f)
or
f = E / (6.626x10^-34 Js)
Finally,
Use
(wavelength)(frequency) = (3.00x10^8m/s)
or
wavelength = (3.00x10^8m/s) / freq. = ______?
================
Hydrogen Atom - Electron Transitions
Q.
A exited hyrogen atom emits light with a frequency of 1.141 x10^14hz to reach the energy level for which n=4 in what principal quantum level did the electron begin.
A.
A exited hyrogen atom emits light with a frequency of 1.141 x10^14hz to reach the energy level for which n=4 in what principal quantum level did the electron begin
The energy of the photon with a given frequency, f, is:
E = hf
For this example,
E = (6.626x10^-34 Js)(1.141 x10^14 hz) = _______?
Also,
E = E(higher) - E(lower)
or
E = Ex - E4 x is the higher level
or
E = E(higher) - (-2.179x10^-18 J / 4^2
or
E(higher) = (2.179x10^-18 J / 4^2) - E
Substitute E from the previous step and solve to get E(higher)
Finally,
E(higher) = (2.179x10^-18 J / x^2)
or
x^2 = 2.179x10^-18 J / [E(higher)] = ________?
==================
A exited hyrogen atom emits light with a frequency of 1.141 x10^14hz to reach the energy level for which n=4 in what principal quantum level did the electron begin.
A.
A exited hyrogen atom emits light with a frequency of 1.141 x10^14hz to reach the energy level for which n=4 in what principal quantum level did the electron begin
The energy of the photon with a given frequency, f, is:
E = hf
For this example,
E = (6.626x10^-34 Js)(1.141 x10^14 hz) = _______?
Also,
E = E(higher) - E(lower)
or
E = Ex - E4 x is the higher level
or
E = E(higher) - (-2.179x10^-18 J / 4^2
or
E(higher) = (2.179x10^-18 J / 4^2) - E
Substitute E from the previous step and solve to get E(higher)
Finally,
E(higher) = (2.179x10^-18 J / x^2)
or
x^2 = 2.179x10^-18 J / [E(higher)] = ________?
==================
11/9/07
Photosynthesis - Mole Ratios
Q.
In photosynthesis, CO2 (g) combines with H2O (g) to form glucose and oxygen. How many moles of glucose would be produced if a) 5 mol of CO2 (g) were used? b) 3 mol of H2O (g) were used? c) 18 mol of O2 (g) were released. My teacher told me to set this up as a math ratio to figure it out: 6 mol CO2 (g) = 5 mol CO2 (g) 1 mol C6H12O6 (s) ? C6H12O6 (s)
A.
The chemical equation is:
6CO2 + 6H2O --–> C6H12O6 + 6O2
(a) (5 mol CO2)( 1 mol C6H12O6 / 6 mol CO2) = 5/6 moles C6H12O6 or 0.833 mol CO2
(Moles CO2 canceling out, mole C6H12O6 remains)
(c) (18 mol O2)(1 mol C6H12O6 / 6 mol O2) = 3 moles C6H12O6
I am showing you above how the coefficients in the balanced chemical equation are used to set up the solutions of parts (a) and (c). Study them carefully and do the other parts in a similar way. If they don’t make sense find a chemistry textbook that explains chemical calculations based on a chemical reaction (two-substance stoichiometry). Chemical calculations of this type are an entire unit of study in a chemistry class. Hard to cover them in an email.
==================
In photosynthesis, CO2 (g) combines with H2O (g) to form glucose and oxygen. How many moles of glucose would be produced if a) 5 mol of CO2 (g) were used? b) 3 mol of H2O (g) were used? c) 18 mol of O2 (g) were released. My teacher told me to set this up as a math ratio to figure it out: 6 mol CO2 (g) = 5 mol CO2 (g) 1 mol C6H12O6 (s) ? C6H12O6 (s)
A.
The chemical equation is:
6CO2 + 6H2O --–> C6H12O6 + 6O2
(a) (5 mol CO2)( 1 mol C6H12O6 / 6 mol CO2) = 5/6 moles C6H12O6 or 0.833 mol CO2
(Moles CO2 canceling out, mole C6H12O6 remains)
(c) (18 mol O2)(1 mol C6H12O6 / 6 mol O2) = 3 moles C6H12O6
I am showing you above how the coefficients in the balanced chemical equation are used to set up the solutions of parts (a) and (c). Study them carefully and do the other parts in a similar way. If they don’t make sense find a chemistry textbook that explains chemical calculations based on a chemical reaction (two-substance stoichiometry). Chemical calculations of this type are an entire unit of study in a chemistry class. Hard to cover them in an email.
==================
Solubility of CO2 in Water
Q.
Solubility Product for CO2(g)<-> CO2(aq) at 25 degrees celcius is calculated to be K = 5.23x10^-2. If the the CO2(aq) in a lake at the same temperature is 2.2mg/L, is the lake in equilibrium with CO2(g)(partial pressure = 10^-3.5? If not in equilibrium, is the gas volatizing from the lake or dissolving into it? CALCULATE Q AND COMPARE IT TO K=5.23X10^-2 TO DETERMINE EQUILIBRIUM. PLEASE SHOW ME HOW TO CALCULATE Q with CO2(g)<->CO(aq). Given: CO2(aq)=2.2mg/L CO2(g) partial pressure =10^-3.5
A.
2.2mg CO2/L = 0.0022g CO2/L
[0.0022g CO2/L]/(44g CO2/mol) = 5.0x10^-5 mol CO2/L
K = pCO2 / [CO2] = 5.23x10^-2
If you substitute NON equilibrium concentrations into the equilibrium constant expression, the value you get is called Q in order to distinguish it from the equilibrium value of K.
Q = pCO2 / [CO2] when pCO2 and/or [CO2] are not at equilibrium
Q = (5.0x10^-5 mol CO2/L) / 10^-3.5 = 0.16
Since Q is larger than K, 0.16 > 5.23x10^-2, the equilibrium,
CO2(g)<-> CO2(aq)
will shift to the right
CO2 gas will dissolve to form more CO2(aq)
=======================
Solubility Product for CO2(g)<-> CO2(aq) at 25 degrees celcius is calculated to be K = 5.23x10^-2. If the the CO2(aq) in a lake at the same temperature is 2.2mg/L, is the lake in equilibrium with CO2(g)(partial pressure = 10^-3.5? If not in equilibrium, is the gas volatizing from the lake or dissolving into it? CALCULATE Q AND COMPARE IT TO K=5.23X10^-2 TO DETERMINE EQUILIBRIUM. PLEASE SHOW ME HOW TO CALCULATE Q with CO2(g)<->CO(aq). Given: CO2(aq)=2.2mg/L CO2(g) partial pressure =10^-3.5
A.
2.2mg CO2/L = 0.0022g CO2/L
[0.0022g CO2/L]/(44g CO2/mol) = 5.0x10^-5 mol CO2/L
K = pCO2 / [CO2] = 5.23x10^-2
If you substitute NON equilibrium concentrations into the equilibrium constant expression, the value you get is called Q in order to distinguish it from the equilibrium value of K.
Q = pCO2 / [CO2] when pCO2 and/or [CO2] are not at equilibrium
Q = (5.0x10^-5 mol CO2/L) / 10^-3.5 = 0.16
Since Q is larger than K, 0.16 > 5.23x10^-2, the equilibrium,
CO2(g)<-> CO2(aq)
will shift to the right
CO2 gas will dissolve to form more CO2(aq)
=======================
Neutralization Reactions
Q.
Write balanced chemical equations for the neutralization reactions: a) hydrochloric acid and sodium hydroxide b) hydrochloric acid and potassium hydroxide c) nitric acid and sodium hydroxide.
A.
The chemical equations are:
(a) NaOH + HCl ---> H2O + NaCl
(b) KOH + HCl ---> H2O + KCl
(c) NaOH + HNO3 ---> H2O + NaNO3
(d) KHCO3 + H2SO4 ---> H2O + CO2 + K2SO4
=================
Write balanced chemical equations for the neutralization reactions: a) hydrochloric acid and sodium hydroxide b) hydrochloric acid and potassium hydroxide c) nitric acid and sodium hydroxide.
A.
The chemical equations are:
(a) NaOH + HCl ---> H2O + NaCl
(b) KOH + HCl ---> H2O + KCl
(c) NaOH + HNO3 ---> H2O + NaNO3
(d) KHCO3 + H2SO4 ---> H2O + CO2 + K2SO4
=================
Oxygen Depletion by Organic Waste
Q.
Upstream of a sewage outfall, a river contains 7mg/liter Dissolved Oxygen. Some distance downstream of the outfall, however, dissolved oxygen has been diminished to 4mg/L due to organic waste decomposition by microbes. What is the approximate amount of organic matter (CH2O) that must have been degraded to account for the this consumption of dissolved oxygen? Hint: Oxidation of organic material by organisms during respiration given by: CH2O + O2 -> CO2 + H2O Not sure how to set up this problem or what formula to use.
A.
1 mole of CH2O = 30 grams
1 mole of O2 = 32 grams
The mass ratio of CH2O to O2 is 30/32 or 0.94 gram organic matter / 1.00 gram O2. The ratio of 0.94 / 1.00 does not depend on the units used. It could be milligrams, grams, etc. The change in DO is 7mg/L – 4mg/L = 3mg/L dissolved O2.
To find the milligrams of organic matter degraded:
(3mg/L dissolved O2)(0.94g organic matter / 1 g O2) = 2.82g organic matter / liter reacted with dissolved oxygen.
[Please note that the milligrams of O2 used and of organic matter degraded are about the same: 3mg and 2.8mg.]
=================
Upstream of a sewage outfall, a river contains 7mg/liter Dissolved Oxygen. Some distance downstream of the outfall, however, dissolved oxygen has been diminished to 4mg/L due to organic waste decomposition by microbes. What is the approximate amount of organic matter (CH2O) that must have been degraded to account for the this consumption of dissolved oxygen? Hint: Oxidation of organic material by organisms during respiration given by: CH2O + O2 -> CO2 + H2O Not sure how to set up this problem or what formula to use.
A.
1 mole of CH2O = 30 grams
1 mole of O2 = 32 grams
The mass ratio of CH2O to O2 is 30/32 or 0.94 gram organic matter / 1.00 gram O2. The ratio of 0.94 / 1.00 does not depend on the units used. It could be milligrams, grams, etc. The change in DO is 7mg/L – 4mg/L = 3mg/L dissolved O2.
To find the milligrams of organic matter degraded:
(3mg/L dissolved O2)(0.94g organic matter / 1 g O2) = 2.82g organic matter / liter reacted with dissolved oxygen.
[Please note that the milligrams of O2 used and of organic matter degraded are about the same: 3mg and 2.8mg.]
=================
Air Pressure and Humidity
Q.
How are air pressure humidity and temperture related?
A.
Air pressure is higher when both, the humidity and the temperature are low. Water vapor has lower density than air. Cold air is denser than warm air.
================
How are air pressure humidity and temperture related?
A.
Air pressure is higher when both, the humidity and the temperature are low. Water vapor has lower density than air. Cold air is denser than warm air.
================
Radical Empiricism
Q.
Radical empiricist proclaim that nothing exists unless we can scientifically measure it and evaluate it. How important is this assumption in the scientific method? What are the strengths and weaknesses of this assumption? What ethical,philosophical, religious, and practical problems does this pose for science in our current world?
A.
Not sure you are representing Radical Empiricism fully or correctly. Does it deal with EVERYTHING? It would HAVE to deal with everything in order to proclaim that “nothing exists unless....”. You may want to read “The Meaning of Truth” by William James who is credited with creating radical empiricism. You can’t reduce an entire philosophical movement to one sentence.
“The postulate is that ‘the only things that shall be debatable among philosophers shall be things definable in terms drawn from experience’”. That statement by William James has to do with searching for the truth, not defining existence. This “truth” refers to a context which includes the physical world but does not necessarily extend beyond it.
The strengths and weaknesses of the alleged assumption you refer to depend on whether it is applied correctly to the areas and contexts for which it was intended. You may want to find out what those are. Are the postulates of empiricism intended for ethical and and religious application?
A question such as the one you sent us is intended for extensive study and research prior to expressing opinions. You can use a search engine with search phrases like “radical empiricism” to lead you to online articles and book references which you may find in your local library. Check:
http://en.wikipedia.org/wiki/Radical_empiricism
http://www.philosophypages.com/hy/4t.htm
http://www.tektonics.org/guest/pslockhume.htm
Radical empiricist proclaim that nothing exists unless we can scientifically measure it and evaluate it. How important is this assumption in the scientific method? What are the strengths and weaknesses of this assumption? What ethical,philosophical, religious, and practical problems does this pose for science in our current world?
A.
Not sure you are representing Radical Empiricism fully or correctly. Does it deal with EVERYTHING? It would HAVE to deal with everything in order to proclaim that “nothing exists unless....”. You may want to read “The Meaning of Truth” by William James who is credited with creating radical empiricism. You can’t reduce an entire philosophical movement to one sentence.
“The postulate is that ‘the only things that shall be debatable among philosophers shall be things definable in terms drawn from experience’”. That statement by William James has to do with searching for the truth, not defining existence. This “truth” refers to a context which includes the physical world but does not necessarily extend beyond it.
The strengths and weaknesses of the alleged assumption you refer to depend on whether it is applied correctly to the areas and contexts for which it was intended. You may want to find out what those are. Are the postulates of empiricism intended for ethical and and religious application?
A question such as the one you sent us is intended for extensive study and research prior to expressing opinions. You can use a search engine with search phrases like “radical empiricism” to lead you to online articles and book references which you may find in your local library. Check:
http://en.wikipedia.org/wiki/Radical_empiricism
http://www.philosophypages.com/hy/4t.htm
http://www.tektonics.org/guest/pslockhume.htm
Newton's 2nd Law
Q.
This question has been submitted in the following subject: science How much force must a pitcher exert on a 0.5 kg baseball in order to accelerate it at 50 m/s2?
A.
F = ma
F = (0.5kg)(50m/s2) = 25 N
===============
This question has been submitted in the following subject: science How much force must a pitcher exert on a 0.5 kg baseball in order to accelerate it at 50 m/s2?
A.
F = ma
F = (0.5kg)(50m/s2) = 25 N
===============
Energy Transformations
Q.
Give an example of each of these energy transformations: kinetic to thermal gravitational to thermal thermal to kinetic
A.
When you drill a hole using an electric drill, the drill bit gets hot. The rotation of the drill bit involves kinetic energy which is converted to heat energy.
When a meteorite falls into the earth’s surface because of the gravitational pull of the earth, heat is released and the meteorite glows as a “shooting star”. Gravitational energy has been converted to heat.
Gasoline burns in a lawn mower engine causing parts of the engine to move. Heat produces motion.
=============
Give an example of each of these energy transformations: kinetic to thermal gravitational to thermal thermal to kinetic
A.
When you drill a hole using an electric drill, the drill bit gets hot. The rotation of the drill bit involves kinetic energy which is converted to heat energy.
When a meteorite falls into the earth’s surface because of the gravitational pull of the earth, heat is released and the meteorite glows as a “shooting star”. Gravitational energy has been converted to heat.
Gasoline burns in a lawn mower engine causing parts of the engine to move. Heat produces motion.
=============
pH and Ionic Strength
Q
A body of water already at equilbrium contains 10^-5 mol/litter of carbonic acid, H2CO3. a)What is the concentration of bicarbonate, HCO3^-, at pH values of 4, 7, and 10. b)Use the same pH conditions in part a) but in seawater having an ionic strength of 0.5M. (Because pH is properly defined on the basis of hydrogen activity, ionic strength corrections need only be made for HCO3^-.) Not sure what to do. There should be an answer for each pH value.
-----------------------
(a)
Ki = [H+][HCO3-] / [H2CO3] = 4.3x10^-7 (from a table of Ka values)
For a pH of 4, [H+] = 1x10^-4
[1x10^-4][HCO3-] / [(1x10^-5)] = 4.3x10^-7
[HCO3-] = [H2CO3](Ka) / [H+]
[HCO3-] = (1x10^-5)(4.3x10^-7) / (1x10^-4) = 4.3x10^-8
You find [HCO3-] for other pH’s in a similar way. As the pH rises, the concentration of HCO3- should also rise.
(b)
Reference:
http://www.chembuddy.com/?left=pH-calculation&right=ionic-strength-activity-coefficients
The hydrogen activity coefficient can be calculated by:
Log(f) = [(-.51z^2)*sqrt(I)] / [1 + sqrt(I)] z = ionic charge, I = ionic strength
Log(f) = -0.21125 and f = 0.615
The hydrogen activity is
a = (f)[H+]
For pH = 4,
a = {H+} = (0.615)(1x10^-4) = 6.15x10^-5 <--- H+ activity replacing [H+]
[HCO3-] = [H2CO3](Ka) / [H+]
[HCO3-] = (1x10^-5)(4.3x10^-7) / (6.15x10^-5) = 7.0x10^-8
For other pH’s, do similar calculations with the same activity coefficient.
GK
A body of water already at equilbrium contains 10^-5 mol/litter of carbonic acid, H2CO3. a)What is the concentration of bicarbonate, HCO3^-, at pH values of 4, 7, and 10. b)Use the same pH conditions in part a) but in seawater having an ionic strength of 0.5M. (Because pH is properly defined on the basis of hydrogen activity, ionic strength corrections need only be made for HCO3^-.) Not sure what to do. There should be an answer for each pH value.
-----------------------
(a)
Ki = [H+][HCO3-] / [H2CO3] = 4.3x10^-7 (from a table of Ka values)
For a pH of 4, [H+] = 1x10^-4
[1x10^-4][HCO3-] / [(1x10^-5)] = 4.3x10^-7
[HCO3-] = [H2CO3](Ka) / [H+]
[HCO3-] = (1x10^-5)(4.3x10^-7) / (1x10^-4) = 4.3x10^-8
You find [HCO3-] for other pH’s in a similar way. As the pH rises, the concentration of HCO3- should also rise.
(b)
Reference:
http://www.chembuddy.com/?left
The hydrogen activity coefficient can be calculated by:
Log(f) = [(-.51z^2)*sqrt(I)] / [1 + sqrt(I)] z = ionic charge, I = ionic strength
Log(f) = -0.21125 and f = 0.615
The hydrogen activity is
a = (f)[H+]
For pH = 4,
a = {H+} = (0.615)(1x10^-4) = 6.15x10^-5 <--- H+ activity replacing [H+]
[HCO3-] = [H2CO3](Ka) / [H+]
[HCO3-] = (1x10^-5)(4.3x10^-7) / (6.15x10^-5) = 7.0x10^-8
For other pH’s, do similar calculations with the same activity coefficient.
GK
Zero Net Force
Q
A car weighing 8000 N moves along a straight, level road at a steady 80 km/hr. The total resistive force on the car is 500 N. What is the drive force on the car?
-----------------------------
If the acceleration is zero, the net force on the car is also zero:
F(drive) + F(resistive) = 0
The resistive force is –500N. I will let you figure out what the drive force must be.
NOTE: The 8000N force of the car’s weight is perpendicular to the direction of motion and it is cancelled out by the resistance of the road. You can ignore it.
GK
------------------------------
A car weighing 8000 N moves along a straight, level road at a steady 80 km/hr. The total resistive force on the car is 500 N. What is the drive force on the car?
-----------------------------
If the acceleration is zero, the net force on the car is also zero:
F(drive) + F(resistive) = 0
The resistive force is –500N. I will let you figure out what the drive force must be.
NOTE: The 8000N force of the car’s weight is perpendicular to the direction of motion and it is cancelled out by the resistance of the road. You can ignore it.
GK
------------------------------
Isomers
Q
Is cyclopentane an isomer of pentane?
----------------
Cyclopentane has the molecular formula C5H10. Pentane has the molecular formula C5H12. The two compounds are not isomers.
GK
----------------
Is cyclopentane an isomer of pentane?
----------------
Cyclopentane has the molecular formula C5H10. Pentane has the molecular formula C5H12. The two compounds are not isomers.
GK
----------------
Subscribe to:
Comments (Atom)
