Use a molecular-orbital analysis to predict which species in each of the following pairs has the stronger bonding between atoms.
A.
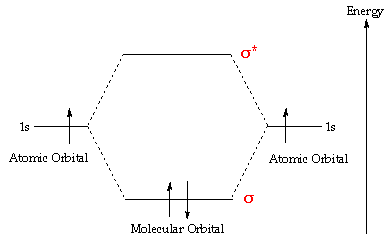
The s-orbitals of the two H atoms merge to form one bonding orbital, sigma-1 and one antibonding orbital, sigma-1*. The strongest bonding between the two hydrogen atoms exists when we have 2 electrons in the sigma-1 bonding orbital and no electrons in the sigma-1* orbital.
The H 2 molecule has 2 electrons in the sigma-1s bonding orbital which fills before the sigma-1s* orbital which has higher energy. That is equivalent ot 1 whole covalent bond.
The H 2+ molecule has only 1 electron which goes into the sigma-1s bonding orbital and no electrons in sigma-1s*. That is equivalent to half a covalent bond. That should explain why the H 2 molecule is more stable than the H 2+ ion.
For more on molecular orbitals of diatomic molecules and ions, go to:
http://www.sparknotes.com/chemistry/bonding/molecularorbital/section1.html
http://www.ch.ic.ac.uk/vchemlib/course/mo_theory/main.html
==============

No comments:
Post a Comment